Using Data to Combat Opioid-Related Harms
January 24, 2020
Substance misuse and addictions to alcohol, prescription drugs, and illicit substances are long-standing but growing problems in the United States. Since the opioid epidemic was officially declared a federal public health emergency in October 2017, states have undertaken numerous activities to address opioid-related harms. State and territorial health agencies (S/THAs) were surveyed in February 2019 to understand how data informs these efforts. This brief describes data-based approaches used by 25 S/THAs (n=25) to address four types of opioid-related harms in their jurisdictions: prenatal substance exposure, neonatal abstinence syndrome, adverse childhood experiences, and injection drug use- associated infections.
Agencies are engaging in data-driven decision making to address opioid-related harms.
Agencies are using the data they collect to provide baseline data (90.9%, n=20), guide the formulation of programs and activities (86.4%, n=19), as well as inform opportunities for collaboration with other organizations (86.4%, n=19).
Prenatal Substance Exposure and Neonatal Abstinence Syndrome (NAS)
Prenatal substance exposure and neonatal abstinence syndrome (NAS) are two opioid-related harms that can occur when infants are exposed to opioids in utero. Agencies are addressing these opioid- related harms affecting neonates and infants in several ways. Most agencies are collecting data on prenatal substance exposure (60.9%, n=14) or will be collecting data in the future (21.7%, n=5). More than half of agencies collect maternal and child health data related to opioids and substance exposure. This number should be even higher in the future.
Of the agencies that currently collect data, one-third (33.3%, n=5) also track medication-assisted treatment (MAT) as a potential source of prenatal substance exposure. Half of respondents endorse at least one type of screening tool for prenatal substance exposure—specifically, Finnegan’s Neonatal Abstinence Scoring Tool (27.3%, n=6) and maternal self-reporting and interviews (18.2%, n=4).
While fewer than half (35%, n=7) have mandatory case reporting of NAS, a majority of responding jurisdictions collect data on neonatal abstinence syndrome (75%, n=18)—a harm that has increased in frequency in most jurisdictions in the past year (59.1%, n=13).
Adverse Childhood Experiences (ACEs)
Tracking adverse childhood experiences—traumatic experiences in childhood that increase the risk for negative health conditions and outcomes later in life—includes the measurement of indicators such as household substance misuse.
Almost All Public Health Agencies Are Collecting Data on Several ACEs Indicators (N=25)
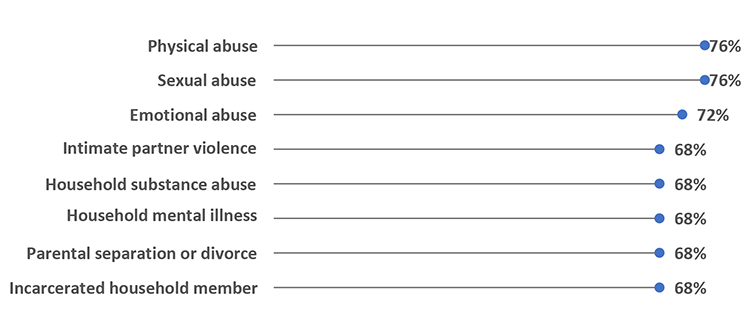
Agencies collect data on ACEs primarily at the individual level (100%, n=21) and often through household surveys (90.5%, n=19).
Injection Drug Use-Associated Infections
The use of injection drugs—increasingly utilized as a vehicle for opioid ingestion—is often associated with blood-borne infections due to unsafe practices like needle sharing. Injection drug use-associated infections are another opioid-related harm being addressed by health agencies.
Over the Past Year, Agencies Reported Seeing Increases in Infections Associated With Injection Drug Use in Their Jurisdictions (N=22)
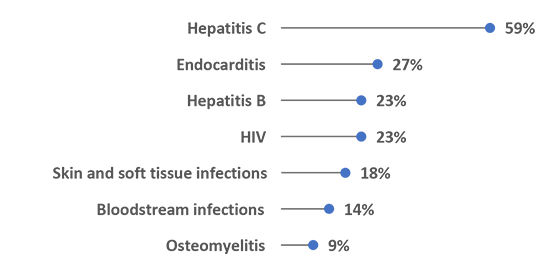
Agencies are working to address this increase in infections associated with injection drug use in their jurisdictions. Almost half of respondents (41.7%, n=10) have a plan in place to address these infections, while an additional 25% (n=6) are actively creating one.
For more information, please email ASTHO’s Research and Evaluation team at researchandevaluation@astho.org. For more information about ASTHO’s opioid response framework, visit my.astho.org/opioids.
This brief was supported by grant or cooperative agreement number NU38OT000161, funded by the Centers for Disease Control and Prevention. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention or the Department of Health and Human Services.