Maximizing the Benefit of COVID-19 Therapeutics: Considerations for State Public Health Officials
April 25, 2022
Introduction
COVID-19 therapeutics offer a critical tool for mitigating COVID-19’s impact on individuals and communities at risk of severe disease and mortality, as well as for reducing the potential impact of future COVID-19 surges on the health system, economy, and communities. COVID-19 therapeutics include monoclonal antibodies (mAbs) and antiviral therapies. These therapeutics have proven effective in reducing hospitalization and death, though new data show some therapeutics have reduced efficacy against Omicron BA.2. The effectiveness of currently distributed (as of mid-April 2022) COVID-19 therapeutics depends on timely provision either prior to exposure (in the case of Evusheld) or within 5-7 days of symptom onset, depending on the therapeutic. The Biden Administration’s National COVID-19 Preparedness Plan includes a nationwide Test to Treat initiative to improve rapid access to oral antiviral therapies. State strategies to facilitate reliable access to testing and treatments will be critical now and in the future to support equitable therapeutic access and uptake.
While states have implemented many strategies for improving equitable testing and therapeutic access, systemic inequities such as limited pharmacy or primary care access continue to prevent historically marginalized populations from receiving therapeutics at rates proportional to COVID-19 cases and mortality. Focusing exclusively on health systems as points of distribution means that many historically marginalized populations may lack access to therapeutics. Reducing inequities in therapeutic access by race, ethnicity, primary language, and socioeconomic status, as well as improving outcomes for individuals with underlying medical conditions associated with a higher risk for severe COVID-19, requires focused efforts to increase the amount of testing and therapeutic resources available to communities experiencing greatest risk; engage and educate providers and communities with current information on therapeutic availability, safety, and eligibility to facilitate utilization; and decrease barriers to testing and therapeutic access through one-stop Test to Treat models or community-based models that extend the reach of conventional health systems.
Effectively deploying COVID-19 therapeutics requires public health officials to navigate shifts in therapeutic supply and demand, logistical challenges in allocating mAbs and antivirals as well as co-locating therapeutics and testing resources, and systemic barriers that have led to underutilization by historically marginalized populations who continue to experience greater risk of severe consequences from COVID-19. This challenge is especially relevant due to greater uncertainty regarding support for these programs when the public health emergency ends. The Duke-Margolis Center for Health Policy and the Association of State and Territorial Health Officials (ASTHO) interviewed state public health officials and reviewed publicly available materials to understand promising approaches to maximize the benefit of therapeutics. This brief highlights promising strategies and considerations to address current challenges in therapeutic distribution and improve timely and equitable access. Common strategies emerged to reach communities who may benefit the most from rapid access to therapeutics. These strategies include:
- Directing therapeutic resources to communities and individuals experiencing highest risk of severe disease.
- Supporting rapid Test to Treat care pathways and reducing barriers to care.
- Increasing provider capacity and awareness of COVID-19 therapeutics.
- Engaging communities and improving public awareness of COVID-19 therapeutics.
State Planning Challenges: Therapeutic Supply Predictability, Transparency, and Coordination
States and territories are responsible for overseeing the allocation of limited therapeutic supplies purchased and distributed by the HHS Assistant Secretary for Preparedness and Response (ASPR). While federal advance purchase agreements have provided states with therapeutic resources to date, supply has often been unpredictable and inadequate to meet demand. The Omicron variant has limited the effectiveness of certain mAbs (see Table 1), complicating supply availability and planning. Additional challenges include 1) federal allocations to states being based on fluctuating case counts and hospitalizations; 2) states having limited visibility into federal allocations to entities such as Indian Health Services, Veterans Affairs, and select health centers supported by the Health Resources & Services Administration (HRSA); and 3) there has been limited planning for, and communication about, longer-term supplies and supports for state distribution of therapeutics—especially after the public health emergency. Thus, state public health officials continue to have limited visibility and predictability, which complicates allocation decisions.
Table 1. Distribution of COVID-19 Therapeutics to States and Territories*
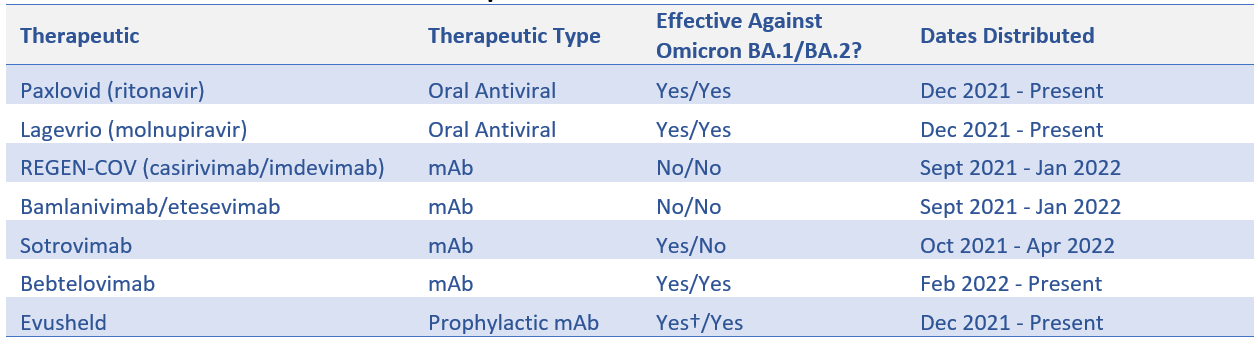
Source: ASPR
* ASPR transitioned to a state- and territory-coordinated distribution system for COVID-19 therapeutics on Sept. 13, 2021.
† On Feb. 24, 2022, the U.S. Food and Drug Administration authorized revisions to Evusheld dosing after data indicated a higher dose may be more likely to prevent BA.1 infection than the originally authorized dose.
Improved predictability of supply, greater transparency, and clearer coordination with federal partners can support state leaders in developing strategies for surging resources to settings and communities that may most benefit from the rapid deployment of therapeutics during outbreaks. As part of their COVID-19 recovery planning efforts, state leaders in California, Oregon, and other states recently announced strategies to ensure adequate supply and access to therapeutic resources to protect people experiencing greatest risk of severe disease and mortality. For example, California’s SMARTER Plan aimed to enable local entities to order and receive effective therapeutics within 48 hours of them becoming available via federal distribution. While therapeutic supply and antiviral availability were initially limited during the December 2021 - February 2022 Omicron surge and have since improved, states should prepare for future surges and the possibility that future variants may limit the effectiveness of existing therapeutic resources while simultaneously increasing demand for treatment.
Promising State Strategies for Improving Equitable Therapeutic Access
Accumulating evidence indicates both underutilization of and inequitable access to COVID-19 therapeutics, though there is a need for more regularly updated utilization data. As of early March, HHS data indicated less than 50% of therapeutic courses distributed to states and territories have been used, with as much as 80% of ordered Evusheld doses unused. Evidence also shows that historically marginalized populations that have borne a disproportionate burden of the COVID-19 pandemic continue to face barriers to treatment access. Recent analysis of mAb utilization data found lower use of mAb treatments among Black, Asian, and other historically marginalized populations relative to non-Hispanic White patients. Additionally, a study of Medicare beneficiaries with a COVID-19 diagnosis found that only 7.2% received mAb treatment between November 2020 and August 2021. In many cases, patients experiencing the highest risk of severe disease were the least likely to receive treatment. Below, we describe promising strategies that have emerged to reach communities who may benefit the most from rapid access to therapeutics.
Direct Therapeutic Resources to Populations Experiencing Highest Risk of Severe Disease
A key challenge for policymakers, health systems, and providers is determining guidelines for eligibility and prioritization of patients experiencing the highest risk of severe COVID-19. To support providers with clinical decision-making, NIH developed guidelines prioritizing patient groups according to factors that include age, vaccination status, immune status, and clinical risk factors for severe COVID-19. Many states and health systems have adopted the NIH guidelines as a decision-making tool for patient eligibility and prioritization. However, additional state efforts have sought to improve equity in therapeutic response by surging therapeutic resources to medically underserved and historically marginalized populations. States also continue to work with providers and community groups to overcome systemic barriers which have contributed to disparities in COVID-19 outcomes. Key strategies for directing therapeutic resources to individuals experiencing highest risk of severe disease include:
- Using disadvantage indices to ensure allocation needs are matched to an effective plan for use: Some states use the CDC/ATSDR Social Vulnerability Index (SVI) to aid in decision-making for prioritizing populations for treatment and to support equitable access to therapeutics for populations that lack access to healthcare resources. Minnesota developed an antiviral allocation strategy based on COVID-19 case counts and SVI data to prioritize patients placed at higher risk. To inform future guidance, the state is working with healthcare systems to facilitate data sharing to analyze patient information to determine if access is equitable. State officials in Connecticut use SVI data to allocate therapeutics to Federally Qualified Health Centers (FQHCs), urgent care centers, and retail pharmacies in areas with high COVID-19 transmission and high levels of social vulnerability. The California Department of Public Health is allocating Paxlovid and Molnupiravir to counties based on weekly incident COVID-19 cases and an equity score based on ZIP-code-level Healthy Places Index (HPI) data. The HPI includes socioeconomic factors that impact health including data on economics, education, transportation, housing, environment, and healthcare access. Oregon is using the COVID-19 Community Vulnerability Index, among other factors, to prioritize allocations of mAbs to communities disproportionately affected by COVID-19.
- Partnering with organizations that serve historically marginalized and medically underserved populations: Community health centers, including FQHCs, provide vital healthcare services to historically marginalized and medically underserved populations, including people who are uninsured, unhoused, have low incomes, or are migrant workers. Many FQHCs receive direct oral antiviral allocations through HRSA’s Health Center COVID-19 Therapeutics Program and will serve as “one stop” clinics as part of the nationwide Test to Treat initiative. Additionally, many states prioritize working with FQHCs and other community clinics to reach populations experiencing high risk of severe COVID-19. For example, Oregon prioritizes FQHCs, tribal clinics, and regional hospitals to provide convenient and equitable access to therapeutics, especially for rural communities. Oregon health officials have indicated intent to re-evaluate allocation criteria and expand partnerships to include more organizations as supply increases. Minnesota works closely with tribal health, correctional settings, and providers serving people experiencing homelessness to provide information and direct allocations of therapeutics. Mississippi created a centers of excellence model in which 40 health systems and clinics, including FQHCs, administer mAbs to patients free of charge. These centers are required to ensure equitable distribution by patient demographics.
Support Rapid Test to Treat Care Pathways and Reduce Barriers to Care
With a limited timeframe for treatment efficacy, facilitating access to COVID-19 testing and treatment through Test to Treat models can reduce delays and barriers to accessing antiviral treatments. While there is no one-size-fits-all approach, Test to Treat models generally seek to co-locate or integrate testing, assessment, and therapeutic dispensing or administration capabilities to reduce barriers between steps along the patient treatment pathway.
The Biden Administration is working to expand the number of one-stop Test to Treat locations, where individuals can be tested, see a healthcare provider, and fill an antiviral prescription within existing retail pharmacy clinics, FQHCs, and long-term care facilities. On March 30, ASPR announced a new, web-based Test to Treat site locator for federally distributed antivirals. Even when physical co-location of testing and therapeutic access may be impractical, many states have taken steps to ensure that individuals testing positive—whether in clinical, community, or at-home settings—are quickly referred to or connected with providers. As at-home testing options continue to proliferate, telehealth will play an expanding role in reducing barriers for individuals who may be homebound or lack physical access to providers. Key strategies to streamline pathways from testing to treatment include:
- Building on COVID-19 testing and vaccination infrastructure: Minnesota prioritized early allocations of antivirals to locations that offer testing, assessment, and therapeutics dispensing, such as large healthcare systems, urgent care clinics, and emergency departments. The state is currently exploring opportunities to expand this model in pharmacies, incorporate at-home testing into treatment pathways, and leverage testing sites for access to antivirals. Connecticut provides antivirals to clinics that have the capacity to assess and test patients and dispense antivirals. Massachusetts has included Evusheld and other mAbs in its at-home COVID-19 vaccination program infrastructure.
- Supporting Test to Treat models at locations accessible to individuals and communities experiencing high risk of severe illness: Several states are piloting Test to Treat models in locations that serve individuals and communities experiencing high risk of severe illness. Oregon is partnering with the Oregon Primary Care Association and three pilot FQHCs to explore how existing infrastructure can be augmented to allow eligible patients to receive evaluation, testing, and treatment all in one visit. Minnesota plans to pilot a Test to Treat model at its state testing sites.
- Using telehealth to facilitate access: Several states are developing telehealth solutions to streamline the process of connecting patients to treatment. Alaska, Massachusetts, and Minnesota are planning to expand telehealth services to facilitate remote patient evaluation.
Increase Provider Capacity and Awareness of COVID-19 Therapeutics
COVID-19 therapeutics have different eligible populations, authorized uses, contraindications, and routes of administration. While states have expanded access to COVID-19 therapeutics in many settings including inpatient, outpatient, community, pharmacy, and at-home, several factors hinder provider willingness to prescribe, administer, or stock COVID-19 therapies. Many states have utilized standing orders to broaden access to mAb therapies and the Biden Administration has extended liability protections for pharmacists administering mAbs under the Public Readiness and Emergency Preparedness Act. However, Emergency Use Authorization language for Molnupiravir and Paxlovid limits prescribing authority to physicians, advanced practice registered nurses, and physicians assistants licensed and authorized under state law due to patient safety concerns and potential drug-drug interactions.
Providers have also experienced logistical and capacity challenges for adopting changes needed to administer mAbs. Providers may need to invest in infusion capabilities, staff training on administration or data reporting, or adjust existing space and workflow to accommodate for infusion or patient monitoring. To bolster capacity, many states work with providers and provider associations to provide training and other support or have established referral networks to allow providers to link patients with available therapeutics. States have also explored running or coordinating community-based sites for mAbs administration to help fill gaps in capacity. The rapid pace of therapeutic development, shifting availability, and continuously evolving guidelines for administration of COVID-19 therapeutics is also a challenge. Limited time to stay current on rapidly evolving information can create lack of awareness of effective therapeutic options or unwillingness to administer therapies. Although this issue is likely to remain a challenge, both ASPR and states have worked to provide regular updates, implementation guides, decision aids, therapeutics locators, and other resources to inform providers. Key state strategies for strengthening provider capacity and awareness include:
- Educating and communicating with healthcare providers about therapeutics, and especially those who care for patients who are at greater risk of severe illness: Updating electronic systems and increasing provider education about therapeutics is essential to help address potential concerns and increase comfort in administering therapeutics. Connecticut, Massachusetts, and Minnesota hold meetings with providers to provide education about therapeutics. Massachusetts provides high-touch engagement with many provider groups and works closely with practices that are interested in administering therapeutics to walk them through the ordering process. Minnesota has also partnered with healthcare systems and regional preparedness coordinators to provide information and support their outreach and communication to patients.
- Using technology to augment capacity: In the context of limited supply or capacity, some states developed technology solutions to make connections to therapeutics available throughout the state. For example, Minnesota established the Minnesota Resource Allocation Platform (MNRAP), an online tool that connects the public and providers with mAbs. After a patient is assessed, providers can use MNRAP to find out where mAbs are available and have a referral sent to an infusion facility. The system will match patients to the closest available appointment. Illinois developed a therapeutics matchmaker website to help redistribute therapeutics from sites that have excess doses to sites that have additional demand.
- Engaging with providers to promote the prophylactic use of Evusheld for populations who may benefit: Currently, Evusheld is the only therapeutic authorized for prophylactic use for an estimated seven million individuals who are immunocompromised and may be unable to mount an adequate response to COVID-19 vaccination. Evusheld remains underutilized due to a variety of challenges, including a lack of provider and patient awareness. States are working with healthcare providers, especially specialists, to facilitate access to, and uptake of, Evusheld for immunocompromised individuals. Providers such as oncologists, rheumatologists, and HIV specialists likely have established relationships with many immunocompromised patients and can recommend or prescribe Evusheld. Once Evusheld became available, Connecticut reached out to immunocompromised residents through physician practices to inform them about Evusheld.
Engage Communities and Improve Public Awareness of COVID-19 Therapeutics
A lack of public awareness about available therapeutics as well as hesitancy to use them are key barriers to access for many individuals who may benefit from treatment. With the efficacy of COVID-19 therapeutics contingent on treatment within a few days of symptom onset, it is important to provide consumers with information on how to recognize symptoms, access testing resources, determine whether they are eligible for therapeutics, and seek out treatment. States are also partnering with testing sites and other community partners to ensure that individuals who receive a positive test (whether at home or in a community or clinical setting) receive information or direct referrals for where they may be able to access treatment resources. Key strategies to engage communities and raise public awareness of therapeutics include:
- Creating public communications campaigns that raise awareness: States have expressed that limited communications about available therapeutics have led to lack of awareness of treatment options among the public. State vendors in Massachusetts and Minnesota distribute information about therapeutics at the state’s community testing sites, and the Minnesota Department of Health (MDH) has contracts with diverse media vendors to share information more broadly in communities, including communities with limited English proficiency. Oregon also has strong relationships with two local community-based organizations, 211info and Vive NW, which allow the public to call to receive information and connect to resources in English and Spanish, respectively. The Alaska Department of Health and Social Services has a COVID hotline that residents can call with questions about therapeutics. Massachusetts is launching a statewide public awareness campaign about therapeutics to share information about their benefits and how to access them and has used social media, online state webpages, and community flyers to spread awareness.
- Ensuring communications are accessible: Ensuring accessible and culturally-informed communications is a key strategy for engaging communities that face systemic barriers. Oregon Health Authority (OHA) launched a patient-facing webpage with information and infographics about COVID-19 treatments (available in 12 languages). OHA also collaborated with accessibility teams to draft scripts to create American Sign Language videos on COVID-19 therapeutics and provide information on COVID-19 therapeutics to OHA’s liaisons for people with disabilities. Minnesota created patient fact sheets about COVID-19 therapeutics available in English, Hmong, Somali, and Spanish.
- Partnering with community-based organizations to conduct outreach and provide information about therapeutics: The Minnesota Department of Health collaborates with its Cultural, Faith, and Disability Communities Branch, which is responsible for building relationships and communicating with historically marginalized populations. MDH also has COVID Community Coordinators, or contracted community-based organizations, that support COVID-19 response efforts in communities and provide information in multiple languages about how to access treatment. Massachusetts plans to use full-time community liaisons from its COVID-19 Vaccine Equity Initiative to provide information about therapeutics to community members at higher risk. Similarly, California is building on its Vaccinate ALL 58 campaign and community engagement efforts to communicate the role, availability, and benefits of timely treatment options.
- Conducting outreach and provide education to people experiencing greater risk of severe illness: Oregon Health Authority created consistent messaging that urges individuals not to go to the emergency room to seek therapeutics. While therapeutics can be accessed in hospitals if necessary, Oregon and Alaska are encouraging patients to speak to their healthcare providers, who can help connect them to therapeutics if they meet eligibility criteria.
Conclusion
Therapeutics will remain an important tool for mitigating harm to communities experiencing highest risk of severe disease. In the event of future surges in cases, additional federal guidance and collaboration could help state leaders prepare to surge capacity to deliver sufficient supplies of tests and therapeutics. To effectively use these therapeutic tools moving forward, state leaders will need to flexibly deploy available resources while working with health system and community leaders to ensure that individuals experiencing highest risk of severe disease can access therapies when needed. State leaders can build on existing community partnerships and infrastructure developed through previous vaccination and testing efforts to increase therapeutic access. Although current data on racial and ethnic disparities in treatment utilization is limited, inequities in access to care and outcomes experienced by historically marginalized populations are likely to persist without focused efforts to reduce barriers for individuals that have been disconnected from the health system. Sustained federal purchases of therapeutics, as well as reimbursement for testing and treatment for individuals who are uninsured, continue to be vital for improving equitable access of treatment services.
Lastly, healthcare payers and health systems will continue to be important partners in advancing effective care models along with federal and state public health officials. As federal and state health officials look beyond the immediate public health emergency, therapeutic distribution will eventually shift toward more traditional direct purchasing and drug reimbursement models. While population-based models and payment incentives can help improve strategies for rapidly connecting populations experiencing highest risk of severe disease with the care that they need, state public health officials will continue to play a key role in ensuring availability and equity of therapeutics.
Contributing Authors
 Katie Greene, Assistant Research Director
Katie Greene, Assistant Research Director
Katie Huber, Policy Analyst
Matt D’Ambrosio, Research Assistant
Andrea Thoumi, Policy Fellow
Mark McClellan, Director

Marcus Plescia, Chief Medical Officer
Jessica Baggett, COVID-19 Response Director
Acknowledgements
Duke-Margolis and ASTHO would like to thank state health officials from Alaska, Connecticut, Massachusetts, Minnesota, Mississippi, and Oregon for participating in interviews and providing feedback to this brief. We would also like to thank Christina Silcox and the COVID-19 Testing Strategies Group at Duke-Margolis for content expertise and logistical assistance. This brief was funded in part by The Rockefeller Foundation.